Advances in stem cell research over the past 15 years have revealed that stem cells constitute the natural repair system of the body.
An in-depth analysis of the scientific literature pertaining to the natural role of stem cells in the body leads to four main conclusions:
- More stem cells in circulation means that more stem cells are available to contribute to tissue repair.
- Increasing the number of circulating stem cells by releasing one’s own stem cells increases the body’s ability to repair.
- The development of degenerative health problems has been linked to a lower number of circulating stem cells.
- Daily stem cell support helps regain and maintain optimal health.
 |
 |
||
CARDIOVASCULAR HEALTHIncreasing the number of circulating stem cells has been shown to support cardiac function and vascular health. |
GLUCOSE METABOLISMIncreasing the number of circulating stem cells was shown to support pancreatic function and healthy glucose levels. |
 |
 |
||
BRAIN HEALTHStem cells can become brain cells and can secrete growth factors that support brain repair. |
TISSUE REPAIRStem cells can transform into cells of virtually any tissue, supporting the overall process of tissue repair. |
||
NUMBER OF CIRCULATING STEM CELLS AND ABILITY TO REPAIR
 |
The number of circulating stem cells was measured in 519 patients with confirmed heart disease, and the occurrence of cardiovascular problems (events) was monitored over one year. The individuals were divided into 3 equal groups: High (BLUE), Average (RED), and Low (GREEN) level of stem cells. The graph represents survival without cardiovascular event over time. Each downward step in the lines indicates an individual that experienced a cardiovascular event. Individuals with fewer circulating stem cells experienced significantly more heart problems when compared with individuals having more circulating stem cells. More stem cells in circulation was associated with a reduced risk of death from cardiovascular causes, a first major cardiovascular event, and hospitalization. The level of circulating stem cells predicted the occurrence of cardiovascular events and death from cardiovascular causes. Werner N, Kosiol S, et al. (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 353(10):999-1007. |
 |
The number of circulating stem cells was quantified in the bloodstream of individuals, within 12 hours of an acute myocardial infarction (AMI). Patients were separated into two groups: 1) individuals with few circulating stem cells and 2) individuals with larger number of circulating stem cells. There was no significant difference in baseline cardiovascular function, such as ejection fraction, between the two groups on the day of the AMI. Six months after the AMI, ejection fraction was significantly better in individuals who had higher baseline levels of circulating stem cells. Conclusion: The study documented a significantly greater improvement in left ventricular function in patients with AMI having higher levels of circulating stem cells. Tomoda H and Aoki N. (2003) Bone marrow stimulation and left ventricular function in acute myocardial infarction. Clin. Cardiol. 26:455-57. |
INCREASING THE NUMBER OF CIRCULATING STEM CELLS ENHANCES THE ABILITY TO REPAIR
 |
Scientists tested the ability of bone marrow stem cells (BMSC), mobilized by cytokines, to home to the infarcted region, replicate, differentiate, and ultimately promote myocardial repair. Heart attack (acute myocardial infarct) was triggered in mice and after recovery, the animals were divided into 2 groups: A) control group, and B) group treated with cytokines in order to trigger Endogenous Stem Cell Mobilization (ESCM). ESCM resulted in a significant degree of tissue regeneration 27 days later. ESCM-induced cardiac repair decreased mortality by 68% and infarct size by 40%. Ejection fraction progressively increased and hemodynamics significantly improved as a consequence of the formation of 15 million new myocytes. In conclusion, ESCM might offer a noninvasive therapeutic strategy for the regeneration of the heart. Orlic D, Kajstura J, Chimenti S, et al. (2001) Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. USA. 98(18):10344-9. |
 |
Scar tissue in the infarcted myocardium and ventricular enlargement are pathological consequences of myocardial infarction that are deleterious to cardiac function. Myocardial infarction was triggered in rats and after recovery, the animals were divided into 2 groups: A) control group, B) group treated with cytokines in order to trigger Endogenous Stem Cell Mobilization (ESCM). ESCM resulted in higher ventricular contractility, lower LV end-diastolic pressure, smaller LV end-diastolic and end-systolic dimensions, and reduced ventricular enlargement compared to control. Conclusion: This study suggests that ESCM could offer beneficial effect for the recovery of myocardial infarction. Sugano Y, Anzai T, Yoshikawa T, et al. (2005) Granulocyte colony-stimulating factor attenuates early ventricular expansion after experimental myocardial infarction. Cardiovasc Res. 1;65(2):446-56. |
 |
Endogenous Stem Cell Mobilization (ESCM) induced by an injection of the cytokine G-SCF was tested and compared to stem cell injection for the treatment of spinal cord injury (SCI) in mice. The graph at the top shows mobility (BBB Score) of the animals as they recover from spinal cord injury. ESCM improved mobility in a way comparable to an injection of bone marrow-derived stem cells within the spinal cord lesion. The picture below shows transection of the spinal cord at various levels in control and ESCM-treated animals. ESCM reduced the atrophy of the spinal cord associated with injury.
Conclusion: ESCM represents a safe and effective treatment modality for SCI, as a viable alternative to injection of bone marrow-derived stem cells. Guo X, Bu X, et al. (2012) Comparison of autologous bone marrow mononuclear cells transplantation and mobilization by granulocyte colony-stimulating factor in experimental spinal injury. International Journal of Neuroscience. 122:723-733. Urdzikova L, Likavcanova-Masinova K., et al. (2011) Flt3 ligand synergizes with granulocyte – colony-stimulating factor in bone marrow mobilization to improve functional outcome after spinal cord injury in the rat. Cytotherapy, 13: 1090–1104. |
 |
Parkinson’s disease was induced in mice by an injection of 6-hydroxydopamine (6-OHDA) in the substantia nigra. The mice were separated into 4 groups: A) sham operated, B) control Parkinson, C) Parkinson treated with L-Dopa and Carbidopa, and D) Parkinson subjected to Endogenous Stem Cell Mobilization (ESCM) induced by an injection of G-CSF. Loss of coordination was quantified by measuring the time taken by the animals to remove a piece of adhesive tape placed on their nose. Control Parkinson’s mice showed a sharp decline in coordination, whose progression was slowed down by L-Dopa and Carbidopa. In addition to a lesser decline in coordination, the animals treated with ESCM showed an apparent recovery after 2 weeks. Prakash A, Chopra K, Medhi B. (2013) Granulocyte-colony stimulating factor improves Parkinson’s disease associated with co-morbid depression: an experimental exploratory study. Indian J Pharmacol. 45(6):612-5. |
FEWER CIRCULATING STEM CELLS IS ASSOCIATED WITH DISEASE DEVELOPMENT
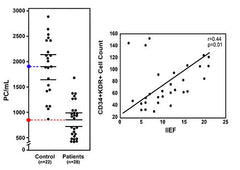 |
The number of circulating stem cells was quantified in the bloodstream of men who complained of erectile dysfunction and was compared to the number of circulating stem cells found in comparable healthy men (control). The number of stem cells circulating in the bloodstream of men complaining of erectile dysfunction was on average slightly less than half the number found in healthy men. When the International Index of Erectile Function (IIEF) was determined in a number of men, a direct relationship was established between erectile function and the number of circulating stem cells. People with more stem cells have better erectile function. Foresta C, Caretta N, et al. (2005) Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 17(3):288-90. Esposito K, Ciotola M, et al. (2009) Circulating CD34+ KDR+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med. 6(1):107-14. |
 |
The number of circulating stem cells was quantified in the bloodstream of diabetic patients suffering from increasing degree of ischemic heart disease (IHD). The frequency of circulating bone marrow-derived progenitor cells is impaired in patients with IHD. The number of circulating stem cells in the bloodstream of individual suffering from stage 3 IHD was roughly half the number found in healthy individuals.
Bozdag-Turan I, Turan RG, Tura nCH, et al. (2011) Relation between the frequency of CD34+ bone marrow derived circulating progenitor cells and the number of diseased coronary arteries in patients with myocardial ischemia and diabetes. Cardiovascular Diabetology 10:107. |
 |
Reduced number and impaired mobilization of endogenous endothelial progenitor cells (EPC) may play an important role in the development of irritable bowel disease (IBD). The number of circulating EPC was quantified in patients suffering from Crohn’s disease (CD) and ulcerative colitis (UC), and compared with control healthy individuals. The number of circulating EPC in CD and UC patients was slightly less than half the number found in healthy individuals.
Garolla A, D’Inca R, Checchin D, et al. (2009) Reduced endothelial progenitor cell number and function in inflammatory bowel disease: a possible link to the pathogenesis. Am J Gastroenterol. 104:2500–2507. |
 |
Systemic lupus erythematosus (SLE) is associated with premature and accelerated atherosclerosis. Circulating progenitor cells (CPCs) are circulating bone-marrow derived cells that play an important role in the repair of vascular damage that underlies the development of atherosclerosis. This study investigated the number and functionality of CPCs in patients with SLE. The number of circulating CPCs as well as their migratory activity were reduced in SLE patients. Conclusion: CPC numbers are reduced in SLE patients and functionality is partly impaired. Moonen JR, de Leeuw K, van Seijen XJ, et al. (2007) Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Research & Therapy 9:R84. |
 |
Endothelial destruction and calcification primarily occur at the aortic side of the calcified aortic valves (AVs). This study investigated whether degenerative AV stenosis (AS) is associated with the presence of valvular endothelial senescence and a reduction in the number and function of endothelial progenitor cells (EPCs). This study found that the number and migratory capacity of EPCs were significantly reduced in AS when compared with control.
Conclusion: Reduced regenerative capacity of valvular ECs due to senescence and decreased levels of EPCs are a pathological link in the destruction of valvular ECs, resulting in progression of degenerative AS. Matsumoto Y, Adams V, Walther C, et al. (2009) Reduced number and function of endothelial progenitor cells in patients with aortic valve stenosis: a novel concept for valvular endothelial cell repair. European Heart Journal 30, 346–355. |
 |
Neurovascular dysfunction and senescent endothelium contribute to the progression of Alzheimer disease (AD). Circulating endothelial progenitor cells (EPCs) provide a cellular reservoir for the endothelial replacement. The levels of EPCs was investigated in patients with AD. AD patients had significantly lower EPC than controls. In patients with AD, a lower EPC level was independently associated with either a lower Mini-Mental State Examination score or a higher Clinical Dementia Rating scale score, indicating a greater reduction in EPC level in advanced AD. Conclusion: Patients with Alzheimer disease (AD) have reduced circulating angiogenic cells. Lee ST, Chu K, Jung KH, Park HK, et al. (2009) Reduced circulating angiogenic cells in Alzheimer disease. Neurology 72:1858–1863 |
 |
Endothelial dysfunction plays a central and critical role in the initiation and development of idiopathic pulmonary arterial hypertension (IPAH) and endothelial progenitor cells (EPCs) play an essential role in endothelium repair. EPCs were isolated and cultured from patients with IPAH and matched healthy volunteers. Circulating EPC numbers as well as migratory and adhesive activity were assessed. A significant decrease was observed in circulating EPC in patients with IPAH. EPCs from patients with IPAH were significantly impaired in their migratory capacity and ability to adhere to fibronectin.
JunHui Z, XingXiang W, GuoSheng F, et al. (2007) Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respiratory Medicine 102, 1073–1079. |
 |
Rheumatoid arthritis (RA) is associated with increased morbidity and mortality attributable to accelerated atherosclerosis and cardiovascular events. This study investigated the link between RA and the number and function of circulating endothelial progenitor cells (EPC). RA was associated with significantly reduced numbers of EPC, decreased migratory activity, and reduced adhesion to mature endothelial cells surface. Conclusion: Endothelial dysfunction in patients with RA, with low grade inflammation, is associated with a reduced number and partial dysfunction of EPC. Herbrig K, Haensel S, Oelschlaegel U, et al. (2006) Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis. 65:157–163. |
 |
Disruption of the endothelial layer is the first step in the development of atherosclerosis. Experimental studies have shown that endothelial progenitor cells (EPCs) are involved in endothelial homeostasis and repair. Conversely, EPC depletion has been associated with atherosclerotic diseases. This study evaluated whether variations in the number of EPCs are associated with subclinical atherosclerosis in healthy subjects, as evaluated by measuring Intima Media Thickness (IMT). Atherosclerotic diseases are associated with higher IMT values. EPCs were significantly reduced in subjects with increased IMT and EPC count was inversely correlated with IMT. Conclusion: Depletion of EPCs is an independent predictor of early subclinical atherosclerosis in healthy subjects. Fadini GP, Coracina a, Baesso I, et al. (2006) Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke 37:2277-2282. |
 |
Chronic renal failure (CRF) has been associated with atherosclerosis and impaired angiogenesis. This study investigated the relation between CRF and EPC number and migration capability. EPCs were isolated from CRF patients on maintenance hemodialysis and from normal control individuals. CRF patients showed markedly decreased numbers of EPC, as well as a decrease in EPC migratory function in response to vascular endothelial growth factor (VEGF). The number of circulating EPC was significantly lower in CRF patients than in normal group under the same burden of risk factors.
Conclusion: EPC biology, which is critical for neovascularization and the maintenance of vascular function, is altered in CRF. Choi JH, Kim KL, Huh W, et al. (2004) Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 24:1246-1252. |
 |
In this study, the numbers and the angiogenic function of EPC from patients with chronic kidney disease (CKD) were assessed in relation to disease progression. Fifty patients with varying degrees of CKD, including 20 patients undergoing dialysis and 10 healthy controls, were included. CKD patients had reduced numbers of circulating CD34+EPC. Furthermore, EPC from patients with CKD displayed functional impairments, i.e., hampered adherence, reduced endothelial outgrowth potential, and reduced antithrombogenic function. These impairments were already observed at stage 1 CKD and became more apparent when CKD progressed.
Conclusion: EPC number and function decrease with advancing CKD, which may hamper physiological vascular repair and can add to the increased risk for cardiovascular diseases observed in CKD patients. Krenning G, Dankers PY, Drouven JW, et al. (2009) Endothelial progenitor cell dysfunction in patients with progressive chronic kidney disease. Am J Physiol Renal Physiol. 296(6):F1314-22. |
 |
Circulating endothelial progenitor cells (EPCs) play a key role in the maintenance of endothelial homoeostasis and promote vascular repair. Reduced EPC number and functional activity have been associated with several cardiovascular risk factors, but their relationship with hypertension remains unclear. This study investigated if number and function of circulating EPCs are reduced in patients with refractory hypertension (RHT). After age adjustment, EPC concentration was significantly reduced in RHT when compared to controls.
Conclusion: The number of circulating EPCs is reduced in patients with RHT and atherosclerotic diseases. Oliveras A, Soler MJ, Martinez-Estrada OM, et al. (2008) Endothelial progenitor cells are reduced in refractory hypertension. Journal of Human Hypertension 22, 183–190. |